TOKYO – Fujifilm’s Avigan could be the first Japanese-developed COVID-19 treatment once it gets approval from the Ministry of Health.
Fujifilm Toyama Chemical Co., Ltd., a subsidiary of Fujifilm Holdings Corp., said Wednesday it will apply for the approval of the antiviral drug for treating new coronavirus patients as early as October after a completed phase III clinical trial, which began in March, showed it can shorten recovery time.
The company announced that of 156 patients, those treated with Avigan improved after 11.9 days compared with 14.7 days for patients who received a placebo.
“No new safety concerns were noted in this trial,” Fujifilm said.
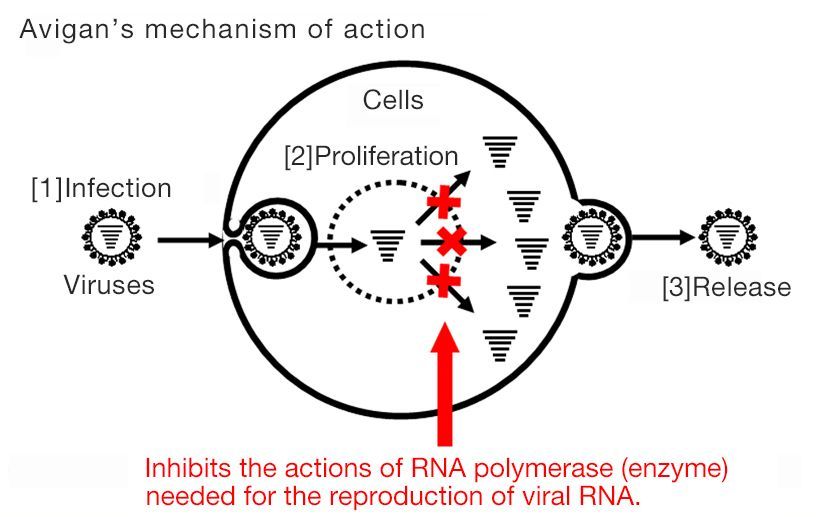
Avigan, also known as favipiravir, was approved for manufacture and sale in Japan in 2014. It specifically blocks RNA polymerase associated with influenza viral replication. This mechanism is expected to have an antiviral effect on SARS-CoV-2, the virus that causes COVID-19.
It was reported to cause side effects on pregnant women.
The Japanese government provided an emergency grant aid of US$1 million for the delivery of Avigan to countries with COVID-19 patients, including the Philippines.
“To meet the requests of the Japanese government to increase stockpiles of Avigan, and by other countries to supply the drug, the Fujifilm Group has been working to increase production of Avigan in collaboration with strategic partners,” the company said.
“The Fujifilm Group will work to deliver the treatment drug to COVID-19 patients as soon as possible, and contribute to ending the spread of COVID-19,” it added. - Florenda Corpuz
(Photographs courtesy of ©FUJIFILM Corporation)


